[ad_1]
By Lambert Strether of Corrente.
In our persevering with concentrate on CDC’s HICPAC (Healthcare An infection Management Practices Advisory Committee), we’ve checked out their upcoming August 22 teleconference, the place it appears doubtless that the conflicted commitee will downgrade HCW safety from airborne ailments from N95s to “Saggy Blues.” On this put up, I wish to take a look at the regulatory auspices beneath which HICPAC conferences are held. Maybe some intelligent lawyer will be capable to work these concepts up into a quick that can stop no matter HICPAC agrees on August from changing into CDC steering.
HICPAC conferences are held, says CDC, beneath the aegis of the Federal Advisory Committee Act (FACA), described by the Congressional Analysis Service (CRO):
Federal advisory committees are created by Congress, Presidents, and govt department businesses to realize experience and coverage recommendation from people exterior the federal authorities. Many federal advisory committees are topic to the Federal Advisory Committee Act (FACA; 5 U.S.C. Chapter 10), which incorporates statutory assembly and transparency necessities. The Committee Administration Secretariat (hereinafter “”Secretariat””) of the Normal Companies Administration (GSA) is chargeable for issues regarding advisory committees topic to FACA. Within the Ultimate Rule, GSA said [w]hile FACA is just not a public participation statute, it immediately impacts how the manager department is held accountable for the use and administration of Federal advisory committees as a serious technique of acquiring public involvement….. To ensure that Congress and the general public to be stored knowledgeable on the actions of advisory committees, FACA supplies that conferences be open and accessible to public inspection. GSA’s dialogue of assembly accessibility contains the requirement that any member of the general public be permitted to file a written assertion with the advisory committee, and be permitted to talk or deal with the advisory committee in accordance with the company’s tips.
That is laudable steering, with which HICPAC is at present out of compliance in three areas: the Minutes, the Agenda, and the “Membership Steadiness Plan.” The primary and third of those violate the letter of FACA; the second violates the spirit. Allow us to take every in flip.
Underneath FACA, HICPAC Assembly Minutes Should Be Posted, However Are Not
In accordance with the GSA (“The Federal Advisory Committee Act (FACA) Brochure“) requires that “federal businesses” (like CDC) “sponsoring advisory committees” (like HICPAC), should “Make accessible for public inspection, topic to the Freedom of Info Act, papers and data, ….” Right here is the HICPAC web site:

The place are the 2023 minutes? (To be truthful, the CRO writes that the GSA’s FACA “steering doesn’t point out that assembly minutes should be printed prematurely of the committee’s subsequent assembly date.” To be truthful, the CDC says though HICPAC conferences might happen “as much as” 8 occasions a 12 months, this 12 months (at the very least in line with the Federal Register) the one earlier 2023 assembly — oddly; was there nothing to debate (in public?) — was on June 8. Certainly 67 days is sufficient time for a big Federal company to organize assembly minutes?)
HICPAC Has Obfuscated the Assembly Agenda, to the Detriment of the Public
In accordance with the CRO, the FACA Ultimate Rule requires advisory committee assembly notices to be printed within the Federal Register at the very least 15 calendar days prematurely of the assembly. The necessities;
the identify of the committee; the time, date, and place of the assembly; a abstract of the agenda; an announcement of whether or not the assembly is open to the general public or shall be closed pursuant to the Authorities within the Sunshine Act (Sunshine Act; 5 U.S.C. §552b); and the identify of the Designated Federal Officer or different accountable company official who could also be contacted for added data in regards to the assembly.
There’s in reality such a Federal Register announcement for HICPAC’s August 22 assembly, and it contains the agenda:
The agenda will embody the next updates: The Healthcare Personnel Guideline Workgroup; Isolation Precautions Guideline Workgroup; Nationwide Healthcare Security Community Workgroup; and Dental Unit Waterlines Guideline Replace. Agenda gadgets are topic to alter as priorities dictate.
(Word the Schrödinger’s Agenda wording on the finish.)
However now let’s check out the HICPAC “Assembly Info” web page at CDC. No agenda:
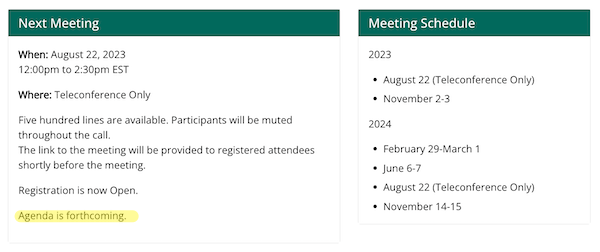
CDC’s personal web site contradict’s its Federal Register announcement. Though presumably the Federal Register is controlling, how is that this “open” or “clear”?
Additional, the “Assembly Info” web page contains the next Kafka-eque registration part:
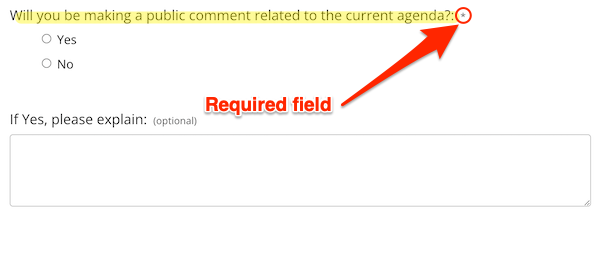
It’s a really good contact to ask a member of the general public in the event that they “shall be making a remark associated to the present agenda” when there isn’t any agenda available! Even higher — shifting into “darkish sample” territory — answering the query is required (by way of the “*”). What’s the person to do? Sort in “Sure, however I don’t know what”? “No, till you inform me what the agenda is”? Abandon the shape in frustration?
Underneath FACA, HICPAC’s “Membership Steadiness Plan” Should Be “Pretty Balanced” in “Factors of View” However Is Not
Right here is the GSA steering on what a “Membership Steadiness Plan” should look like:
Part 5(b)(2) of the FACA requires “”…the membership of the advisory committee to be represented and the features to be carried out by the advisory committee.”” The corresponding FACA laws reiterate this requirement at 41 CFR § 102-3.30(c), and, for discretionary committees being established, renewed, or reestablished, require businesses to offer an outline of their plan to realize pretty balanced membership throughout the constitution session course of with GSA (41 CFR § 102- 3.60(b)(3)). The doc created via this course of is the Membership Steadiness Plan
CDC’s HICPAC does, in reality, have a “Membership Steadiness Plan.” Sadly, it’s not accessible to most of the people. GSA elaborates on factors of view:
The FACA laws supply steering in reaching a balanced Federal advisory committee membership, which embody contemplating: (iii)The forms of particular views required, resembling these of shoppers, technical consultants, the general public at-large, academia, enterprise, or different sectors; (iv) The necessity to acquire on the problems earlier than the Federal advisory committee…
It’s plain as day that HICPAC’s membership is just not “divergent” as FACA understands the time period; each considered one of its members is affiliated both with a hospital or a medical care facility. Conflicts apart, the controlling assumption can solely be that hospital employees don’t have anything to find out about an infection management from anybody exterior their milieu. Certainly such psychological and ideological inbreeding is precisely what an open and clear course of seeks to stop? (“Daylight is the most effective disinfectant,” as they are saying.) CDC has a whole institute, NIOSH, with experience in “respirators and masks.” Are we significantly to consider that NIOSH has nothing to contribute to HICPAC on masking? Or air flow? Or coaching? Even when NIOSH doesn’t sit on the committee, why are they not invited consultants? Why on earth does HICPAC’s draft “Isolation Precautions Guideline Workgroup” deliverable (PDF), which is driving masking coverage and the an infection mannequin for HICPAC, not even point out NIOSH?
An infection Management As we speak feedback:
HICPAC’s composition was a priority for a lot of commentators. As burdened by M.Ok. Fletcher, MSPH, BS, the committee ought to embody “”aerosol scientists and air flow consultants, respirator safety consultants, and industrial hygienists.””
Greater than 900 occupational security, aerosols scientists, public well being, and medical consultants have already written to new CDC director Mandy Cohen telling her that CDC/HICPAC should appropriate their evaluation to mirror the science of aerosols transmission and their decision-making course of to incorporate affected person advocates, aerosols scientists, union representatives and occupational security and well being consultants.
Redress from HICPAC’s Designated Federal Officer?
FACA, says the GSA, requires a “Designated Federal Officer“:
As well as, a Designated Federal Officer should be assigned to every committee to:
- , and another relevant legal guidelines and laws;
- committee conferences;
- Approve agendas;
- Keep required data on prices and membership;
- Guarantee environment friendly operations;
- Keep data for availability to the general public; and
- Present copies of committee reviews to the Committee Administration Officer for forwarding to the Library of Congress.
The CDC says that HICPAC’s Designated Federal Officer is Michael Bell, M.D. From the Federal Register, right here is Bell’s contact data, as of 2020:
FOR FURTHER INFORMATION CONTACT: Michael Bell, M.D., Designated Federal Officer, HICPAC, Division of Healthcare High quality Promotion, Nationwide Middle for Rising and Zoonotic Infectious Illnesses, CDC, l600 Clifton Street, NE, MS H16-3, Atlanta, Georgia 30329-4027; Phone: 404-639-4000; E mail: [email protected].
GSA’s FACA steering, above, permits Bell, in his capability as Designated Federal Officer, to name conferences, and subsequently to not name them (or to right away adjourn them, if known as). I recommend that Bell train his powers to “guarantee compliance with FACA”, and postpone the subsequent HICPAC assembly till (a) the earlier assembly’s agenda is up, (b) the assembly’s agenda is posted on the CDC’s web site (with, ideally, the Schrödinger’s clause eliminated, and (c) HICPAC’s committee composition has “divergent” factors of view.
APPENDIX
In accordance with An infection Management As we speak: “Vital [HICPAC] votes are held earlier than and never after public remark.” If true, that’s each a foul look, and dangerous. The Designated Federal Officer ought to cease this as effectively.
[ad_2]
