[ad_1]
How lengthy does it take totally different international locations/areas to approve new medicines? The US is the quickest and Europe is the slowest amongst chosen main pharmaceutical markets in response to a report by EFPIA.
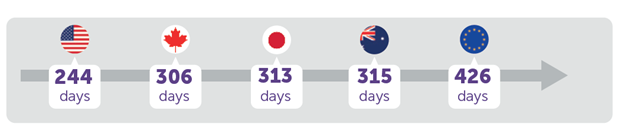
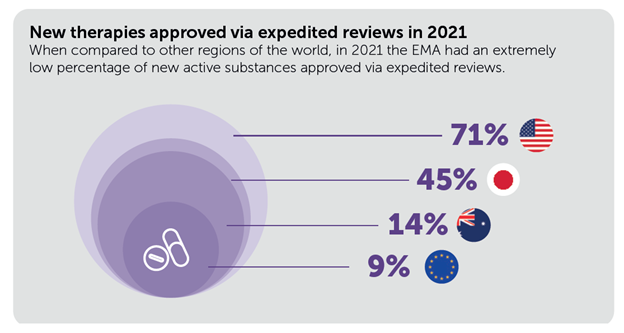
Furthermore, whereas a majority (7 out of 10) of therapies within the US depend on expedited approval, in Europe lower than 1 in 10 are authorized through these pathways.
You may learn EFPIA’s full report and response to the EU pharmaceutical packet right here.
[ad_2]