[ad_1]
Whereas the Inflation Discount Act (IRA) goals to decrease the costs of prescription drugs, on the similar time it exempts medicine treating uncommon ailments (i.e., orphan medicine) from drug worth negotiation. Nonetheless, this exemption is just legitimate if the orphan medicine has a single authorised indication. Thus, a key query is how steadily orphan medicine are later developed for different indications. In that case, IRA may may de-incentivize R&D investments in new indications for orphan medicine.
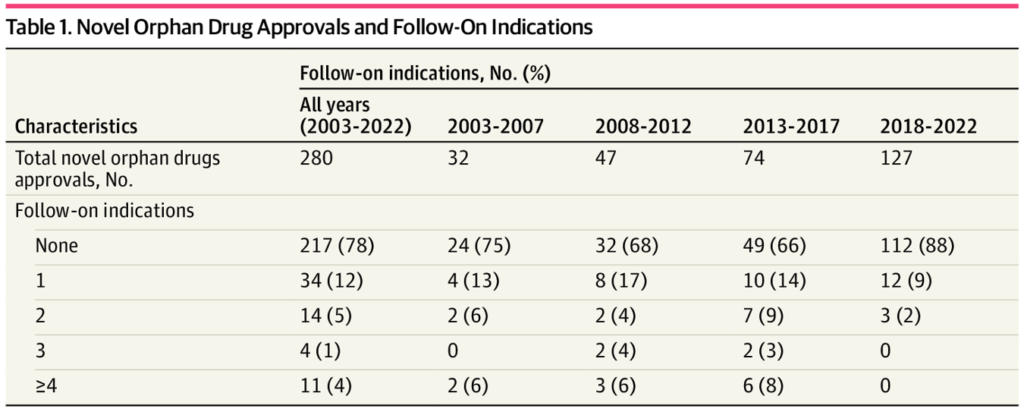
A brand new paper by Chambers et al. (2023) in JAMA Community Open FDA approval database between 2003 and 2022 to see how frequent new indications are for orphan medicine. They discover that:
FDA authorised 282 novel orphan medicine from 2003 to 2022…General, the FDA authorised 152 separate follow-on indications; 92 (61%) of those follow-on indications had been additionally for orphan drug situations. The imply (SD) time from novel orphan drug approval to follow-on indication was 53 (43) months…FDA included 58 (38%) follow-on indications in a single expedited assessment program, 46 (30%) in 2 applications, and 17 (11%) in 3 applications; none had been included in all 4 applications.
You may learn the total article right here.
[ad_2]