[ad_1]
2023 was a robust 12 months for drug discovery. Mullard et al. (2024) report:
The FDA’s Heart for Drug Analysis and Analysis (CDER) authorised 55 new medication in 2023, because the small molecule and biologic pharmacopoeia continues to develop. This cohort is almost 50% larger than the brand new approval class of 2022, which fell under the approval development line. The ten-year rolling common for brand new CDER approvals now stands at 46 per 12 months, the very best it has been in over 20 years. The nadir was 2010, when this common bottomed out at 25 per 12 months.
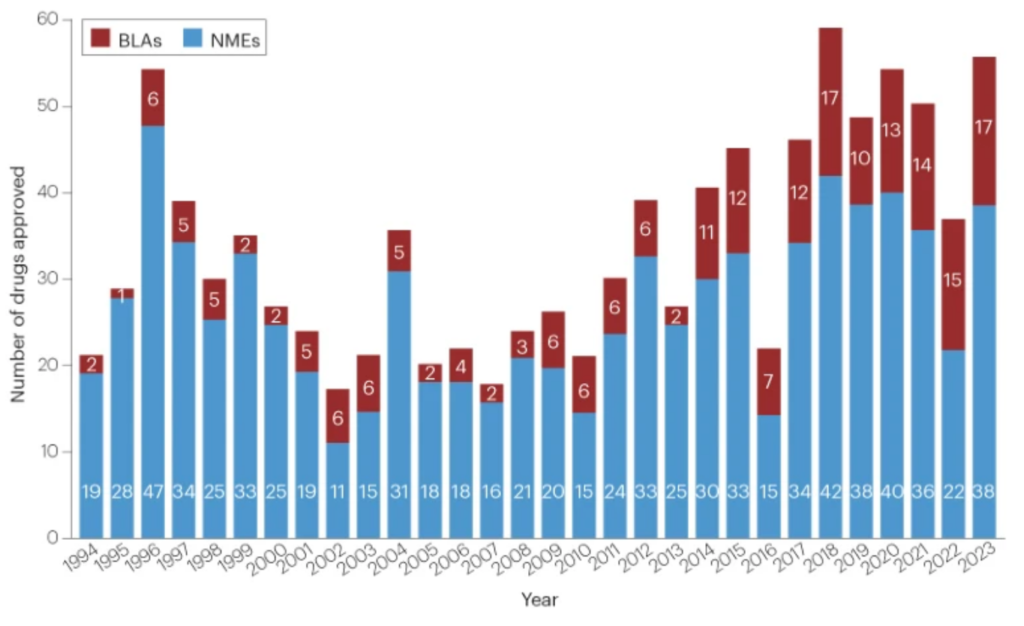
The complete checklist of approvals will be discovered right here.
[ad_2]